7 Hydrogen Isotopes – their Applications, and Key Points
Hydrogen Isotopes
The hydrogen isotopes have, respectively, mass numbers of one, two, and three, and therefore 1H, 2H, and 3H are their nuclear symbols.
Hydrogen has atomic number one and is the first element in the periodic table. The isotopes are those elements that have the same atomic number but a different mass number, and there are three hydrogen isotopes such as protium 1H1, deuterium 1H2, or D and tritium 1H3. Because of the different numbers of neutrons present in them, the isotopes are other.
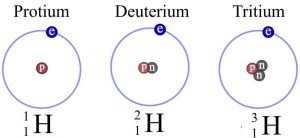
There is no presence of neutrons in protium, and there is one neutron in deuterium and two neutrons in tritium. Protium is the most prominent form of hydrogen, and as deuterium, 0.0156% of hydrogen is present on the earth’s surface. The concentration is one atom per 1018 atoms of protium in tritium.
In hydrogen isotopes, the only tritium is radioactive, which emits low-energy particles. Hydrogen isotopes have a difference in their reaction rates, but they all have similar chemical properties, and the electronic configuration of isotopes is the same. However, because of the significant variations in mass, they have different physical properties.
Due to its light nature, the occurrence of hydrogen on earth is complex, and in plant and animal tissues, hydrocarbons, proteins, hydrides, and many other compounds, hydrogen also occurs. Hydrogen is also the principal element of the solar atmosphere and is the most abundant element in the universe.
Hydrogen Isotopes:
There are the following hydrogen isotopes as given below;
- Protium (1H)
- Deuterium (2H)
- Tritium (3H)
- Hydrogen-4
- Hydrogen-5
- Hydrogen-6
- Hydrogen-7
Protium: (1H)
Protium is one of the common hydrogen isotopes, and it is plenty in nature, with an abundance of 99.98 percent. The reason for this is that the nucleus of this isotope consists of a single proton, and it has been reported to be decayed at no time.
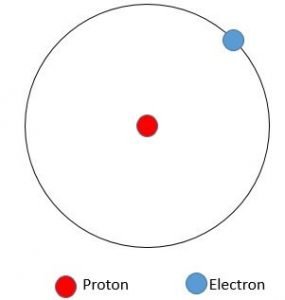
1.007825 amu is the mass of protium, and hydrogen generally combines with other atoms in compounds and is usually found in H2.
Applications of Protium:
There are the following applications of protium such as;
- To prepare certain metals or steels like tungsten in a pure state, protium is a successful minimizing agent.
- In the production of high temperature by suggests of oxyhydrogen flame for welding purposes, it is used.
- In filling balloons and dirigibles, it is an outstanding lifting agent.
Deuterium: (2H)
These hydrogen isotopes have 1 proton and one neutron in their nucleus, and the nucleus of hydrogen two is termed as deuteron. Therefore, it is not radioactive, and for hydrogen 1, its compounds are used in chemical analysis and solvents.
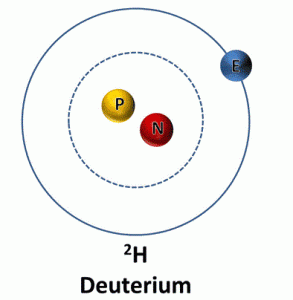
With molecules consisting of deuterium instead of protium, heavy water is enriched, and as a coolant and a neutron moderator, it is used. In nuclear fusion, hydrogen two is also used as a fuel, and as deuterium gas, it occurs naturally.
Applications of Deuterium:
There are the following applications of deuterium such as;
- Drugs and nuclear weapons.
- Contrast properties.
- Tracing and NMR spectroscopy.
- Nuclear Power Plants and atomic reactors.
Tritium: (3H)
Tritium comprises 1 proton and two neutrons in its nucleus, and due to the synergy of cosmic rays with atmospheric gases, tiny traces of hydrogen three or tritium occur in nature.

At the time of nuclear weapons tests, they are also released in a small amount, and it decays into helium 3 through beta decay and is radioactive. 3.0160492 u is the atomic mass of hydrogen 3.
Applications of Tritium:
There are the following applications of tritium such as;
- Analytical chemistry and controlled nuclear fusion.
- Boosting and tritium in hydrogen bomb secondaries.
- Neutron initiator and nuclear weapons.
- Self-powered lighting.
- Used as an oceanic transient tracer.
Hydrogen-4:
Hydrogen-4 contains 1 proton and three neutrons in its nucleus, and these are highly unstable hydrogen isotopes. However, with fast-moving deuterium nuclei, it is incorporated in laboratories bombarding tritium, and 4.02781 ± 0.00011 is the atomic mass of this isotope.
Hydrogen-5:
It consists of 1 proton and four neutrons in its nucleus, and these are highly unstable hydrogen isotopes. By bombarding tritium with fast-moving tritium nuclei, it has been incorporated in the laboratory.
Hydrogen-6:
Hydrogen-6 has a half-life of 290 yoctoseconds, and through triple neutron emission into hydrogen-3, it decays.
Hydrogen-7:
Hydrogen-7 contains 1 proton and six neutrons in its nucleus, and 23 yoctoseconds are the half-life of this.
Key Points:
- With an abundance of 99.98%, the most prevalent hydrogen isotopes are protium and consist of one electron and one proton. Protium is typically not found in its monoatomic form but bonded with other materials or itself.
- Deuterium is a hydrogen isotope consisting of one proton, one electron, and one neutron. In nuclear magnetic resonance studies, it has significant applications.
- Tritium is a hydrogen isotope consisting of one proton, one electron, and two neutrons. It has a half-life of 12.32 years and is radioactive.
